- Case Report
- Open access
- Published:
A case report of fludarabine associated ectopic atrial bradycardia and literature review of fludarabine induced bradycardia
Cardio-Oncology volume 10, Article number: 50 (2024)
Abstract
Background
Fludarabine is a chemotherapeutic agent with lymphodepleting effects that is increasingly used as part of a conditioning regimen prior to allogeneic stem cell transplantation. Fludarabine is generally considered a relatively safe medication with only rare cases of cardiotoxic side effects.
Case Presentation
Here, we present a case of a 30-year-old woman who was undergoing conditioning for a haploidentical cell transplantation for treatment of Fanconi anemia with a 5-day course of daily fludarabine infusion. After her second fludarabine infusion, she was noted to have ectopic atrial bradycardia that resolved with supportive therapy and completion of fludarabine infusion.
Conclusion
We report the first case of ectopic atrial bradycardia associated with fludarabine. Although rare and transient, clinicians should recognize this rare cardiotoxic side effect of fludarabine.
Background
Fludarabine is a synthetic purine analog that is commonly used in the treatment of hematologic malignancies, including chronic lymphocytic leukemia, multiple myeloma, Waldenstrom’s macroglobulinemia, and non-Hodgkin’s Lymphoma [1]. Fludarabine is also used as part of lymphodepleting chemotherapy prior to chimeric antigen receptor (CAR) T cell treatment and as a conditioning agent for allogeneic stem cell transplantation where its immunosuppressive properties facilitate engraftment [2]. The major toxicity of fludarabine is myelosuppression, and a small risk of late myelodysplasia [3]. Although, cardiotoxicity associated with chemotherapeutic agents such as anthracyclines, 5-fluorouracil, and taxanes is well-documented in the literature, fludarabine is considered relatively safe in respect to its effects on the cardiovascular system, with limited number of case reports documenting myocarditis, congestive heart failure, and arrhythmias [4,5,6]. Arrhythmias associated with fludarabine include atrial fibrillation, atrial flutter, supraventricular tachycardia, and sinus bradycardia [4, 7, 8]. Given the paucity of literature on the effects of fludarabine on the cardiovascular system, subtle cardiovascular effects may be missed in clinical practice and under-reported by physicians. Here, we present the first case of fludarabine associated ectopic atrial bradycardia in a young woman undergoing conditioning for haploidentical cell transplantation for the treatment of Fanconi anemia. We also present a review of the current literature on fludarabine induced bradycardia.
Case presentation
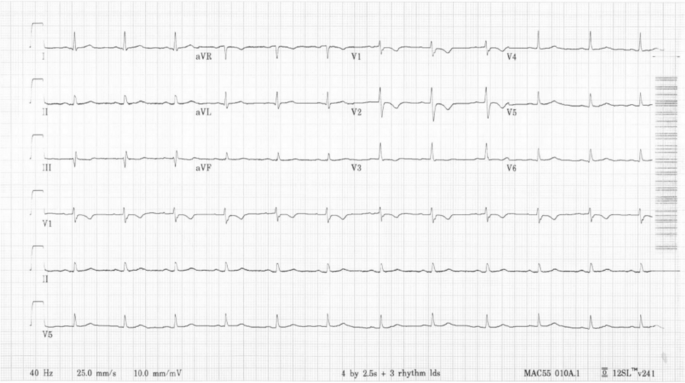
A 30-year-old woman with a history of type 2 diabetes was diagnosed with Fanconi Anemia in December 2022. She had immigrated from the Dominican Republic with a history of long-standing anemia and thrombocytopenia. The patient underwent a bone marrow biopsy in August 2022 that showed hypercellular marrow with dysplastic myeloid precursors, absent megakaryocytes, and karyotypic abnormalities consistent with myelodysplastic syndrome (MDS). The patient underwent chromosome breakage studies and then next generation sequencing studies in December 2022 that confirmed the diagnosis of Fanconi anemia with mutations in the FANCA gene. She was admitted to our hospital for chemotherapy and reduced intensity conditioning haploidentical cell transplant including rabbit anti-thymocyte globulin (ATG), fludarabine, and 300 centigray (cGy) total body irradiation. She underwent a transthoracic echocardiogram that showed normal biventricular size and function with a left ventricular (LV) ejection fraction of 60%. The baseline EKG showed normal sinus rhythm (Fig. 1).
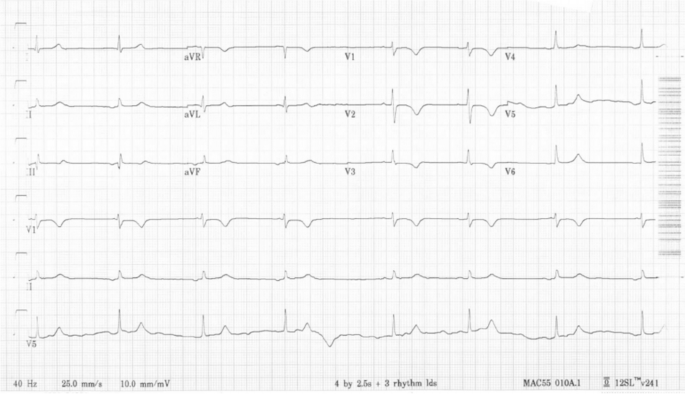
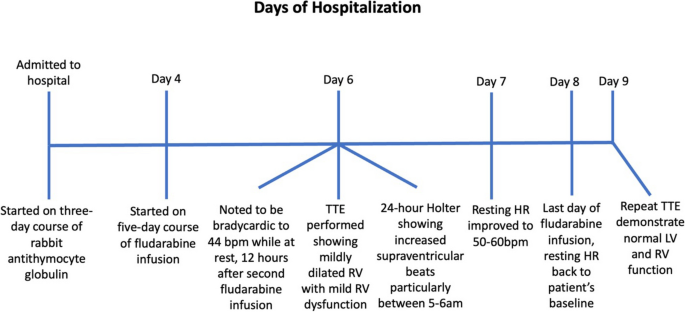
At the time of admission, the patient’s vital signs were within normal limits, blood pressure 114/75 mmHg, heart rate 74 beats per minute (bpm), respiratory rate 18 breaths per minute, and SpO2 100% on room air. During the first three days of her hospital stay, she received daily infusions of rabbit ATG which was associated with fever that resolved with steroids. Subsequently, she was started on a 5-day course of fludarabine infusions at a dose of 30 mg/m2 per day. However, 12 h after the completion of her second fludarabine infusion, the patient was noted to have a heart rate of 40–50 bpm. EKGs showed evidence of ectopic atrial bradycardia with ventricular rate of 44 bpm (Fig. 2), a significant change from her baseline. Other vital signs showed BP 111/74 mmHg, respiratory rate of 18 breaths per minute, and SpO2 of 100% on room air. There were no electrolyte abnormalities noted on her laboratory data. Review of her recent medications did not reveal any agents that could have caused bradycardia. Despite the new onset bradycardia, the patient remained asymptomatic with appropriate chronotropic response with activity, with heart rate that improved to 70–80 bpm with exertion.
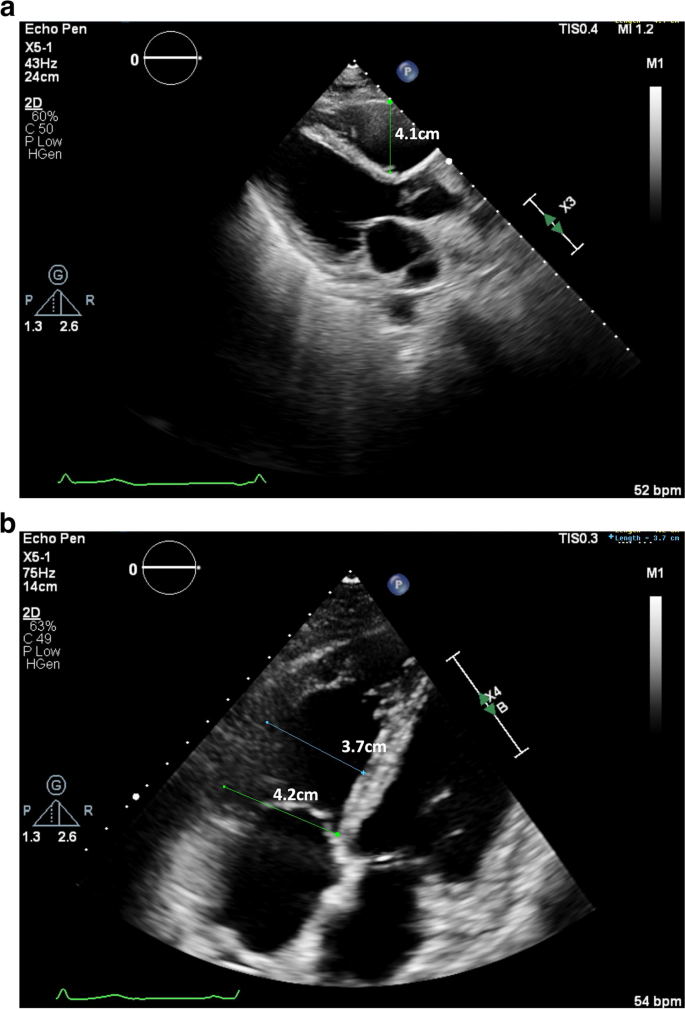
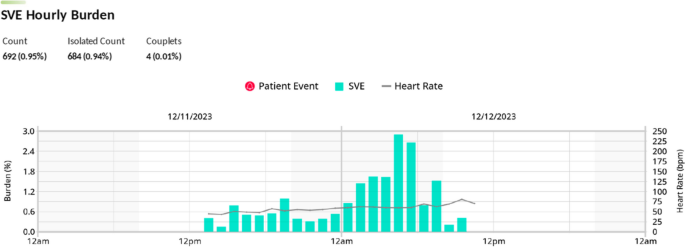
A transthoracic echocardiogram performed on day 6 of hospital course (3 days after starting fludarabine), showed normal LV size and function with an estimated LV ejection fraction of 60%. It also showed a mildly dilated right ventricle (RV) with a proximal right ventricular outflow tract (RVOT) measurement of 4.1 cm on parasternal long axis view (Fig. 3a) and a basal diameter 4.2 cm and mid cavity diameter 3.7 cm on apical 4 chamber view along with mild RV dysfunction (Fig. 3b), which were not previously seen. Decision was made to closely monitor the patient on a Holter monitor and to complete the remaining infusions of fludarabine. A Holter Monitor was used as the Oncology unit did not have the capacity to place her on telemetry. She had a 24-h Holter Monitor placed during hospital day 6, day 3 of fludarabine infusion, that showed increased supraventricular ectopic beats, particularly while the patient was asleep between 5–6 am, 13–14 h after her third fludarabine infusion (Fig. 4).
a and b Parasternal long axis and apical 4 chamber views showing mild RV dilation, with a measured proximal RVOT of 4.1 cm [Upper limit normal < 3 cm] and RV basal diameter 4.2 cm and mid cavity diameter 3.7 cm [Upper limit normal < 4.1 cm and < 3.5 cm respectively] with basal RV hypokinesis on TTE performed on hospital day 6, after 2 doses of fludarabine infusions
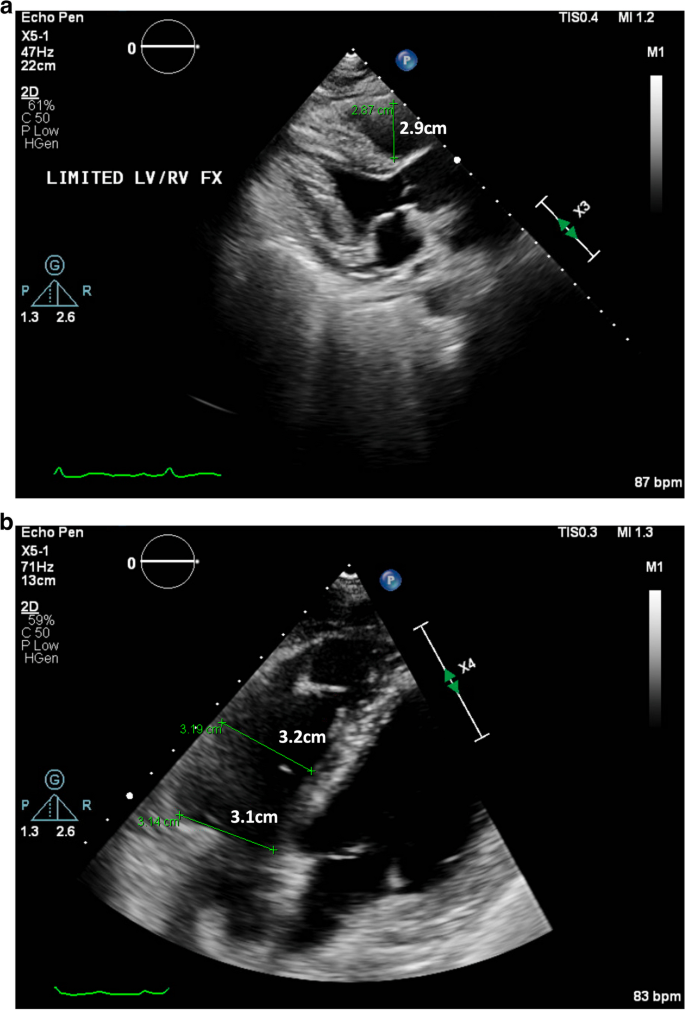
Subsequently, on day 4 of 5 fludarabine infusion, the heart rate improved to 50–60 bpm at rest. On day 5 of 5 of fludarabine infusion, the patient’s resting heart rate had returned to her baseline of 60–70 bpm. A repeat transthoracic echocardiogram one day after the completion of her fludarabine infusions, showed normal LV size and LV ejection fraction of 65% with return of RV size to normal (proximal RVOT measurement of 2.9 cm on parasternal long axis view and basal diameter 3.1 cm, mid cavity diameter 3.2 cm, and longitudinal diameter 6.2 cm on apical 4 chamber view), and no further evidence of RV dysfunction (Fig. 5a and b).
a and b Parasternal long axis and apical 4 chamber views showing normal RV size on repeat echocardiogram on hospital day 9, 1 day after completion of fludarabine infusions, with proximal RVOT measurement of 2.9cm [Upper limit normal <3cm] and RV basal diameter 3.1cm and mid cavity diameter 3.2cm [Upper limit normal <4.1cm and <3.5cm respectively]
Discussion
In this report, we present a case of fludarabine associated ectopic atrial bradycardia in a young woman undergoing conditioning for a haploidentical cell transplantation. Ectopic atrial bradycardia was first observed 12 h after the completion of her second fludarabine infusion. Other causes of bradycardia were excluded, including electrolyte abnormalities, medications, thyroid dysfunction, and ischemia. Patient was noted to have increased supraventricular ectopic beats on a 24-h Holter Monitor, 13–14 h after her third fludarabine infusion. Bradycardia resolved completely by the fifth fludarabine infusion (Fig. 6). She remained asymptomatic through her periods of bradycardia with appropriate chronotropic response to activity. The Adverse Drug Reaction Probability Scale (Naranjo) was calculated to be a total score of 2, suggesting that the bradycardia was a possible adverse drug reaction to Fludarabine.
We conducted a literature search using MEDLINE, journals and manuscripts deposited in PubMed Central, and National Center for Biotechnology Information. A search using the terms “fludarabine” and “bradycardia” resulted in five search results, while a search using “fludarabine” and “heart rate” resulted in three search results. After excluding overlaps and only including results that specified the type of observed bradycardia, a total of three articles met criteria for review.
First article was a case-report published by Chung-Lo et al. in 2010 that reported a 22-year-old male with acute myelogenous leukemia who developed sinus bradycardia after infusion of fludarabine 30 mg/m2 [9]. This patient was scheduled to receive five days of daily infusions of fludarabine. He started experiencing sinus bradycardia at a rate of 45–50 bpm, thirty minutes after the initial fludarabine infusion was started. The patient remained bradycardic until he completed his five-day course of fludarabine.
The second article was a recent retrospective case-series study published by Celik et al. in 2023 that included 73 patients who received fludarabine, of which 13 patients had developed bradycardia [8]. The median time to develop bradycardia after fludarabine administration was 25 min. No pathological findings other than sinus bradycardia were observed. Notably, the only significant difference between patients who developed bradycardia and those who did not was patient age, with the mean age in the bradycardia group being 19 years younger than those without bradycardia. The median age of patients who experienced bradycardia was 34, and the median age of those without bradycardia was 53.
The third article was a prospective cohort study published by Poreba et al. in 2018 in which 56 patients with hematologic malignancies who were to undergo conditioning with high dose chemotherapy followed by hematopoietic stem cell transplantation (HSCT) were placed on a 24-h EKG monitor prior to therapy followed by a post-HSCT 24-h EKG monitor [10]. The results showed that post HSCT had a statistically significant higher percentage of premature ventricular contractions, tachycardia, and Mobitz type 1 s degree atrioventricular block. There was no statistically significant difference in the number of patients who experienced bradycardia. Of the 56 patients, 6 patients underwent chemotherapy conditioning with fludarabine. However, it did not disclose which patients with bradycardia had been treated with fludarabine. Additionally, the type of bradycardia the patients experienced was also not mentioned.
There have been several proposed mechanisms of how different chemotherapeutic agents can contribute to bradycardia, including interference with cardiac conduction, hypersensitivity reaction, stimulation of parasympathetic system, direct toxic and negative chronotropic effects, electrolyte disturbances, and transient ischemia [11,12,13,14]. However, the effect of fludarabine on the cardiovascular system and the mechanism of fludarabine induced bradycardia is not well understood and sparsely reported in the literature. One proposed mechanism suggests that fludarabine is a nucleoside prodrug that has an intermediate metabolite called fluoroadenosine, which may have similar signaling characteristics to adenosine, activating the acetylcholine-gated potassium channel which slows the sinoatrial (SA) node pacemaker rate [8].
The diagnosis of ectopic atrial bradycardia was derived by the morphology of the P waves on the patient’s EKGs. The P waves in leads II, III, and AVF are negatively vectored and positively vectored in lead V1 (Fig. 2). These abnormal P wave vectors are indicative of a P wave that likely originated from outside the SA node. Furthermore, the very short PR intervals on her EKGs also suggest that the P waves are likely originating from outside the SA node. However, another etiology to consider is a junctional escape rhythm with a retrograde atrial activation, in which the retrograde conduction is faster than the antegrade conduction leading to the P wave falling before the QRS complexes.
Importantly, this is the first case of fludarabine associated bradycardia that showed the emergence of an ectopic P wave bradycardia. We hypothesize that when her SA node was slowed by fludarabine, a competing ectopic atrial focus took over the pacing duties, resulting in the ectopic atrial bradycardia that were seen on serial EKGs and the Holter Monitor. Of note, her 24-h Holter Monitor showed that there was an increased presence of supraventricular ectopic beats while the patient was sleeping. This is likely due to the combined effects of fludarabine induced slowing of the SA node and the increased parasympathetic tone during sleep, leading to the ectopic atrial focus to take over as the pacing cells [15].
Our patient was also noted to have mild RV dilation with mild RV dysfunction after two doses of fludarabine, which was not present in her baseline transthoracic echocardiogram. Interestingly, this RV dysfunction was no longer present on a repeated transthoracic echocardiogram performed one day after the completion of her fludarabine infusion. We hypothesize that this transient RV dysfunction was caused by increased loading conditions from large volume infusions as a part of the chemotherapy protocol. However, direct effects of fludarabine cannot be excluded. Fludarabine induced cardiomyopathy is thought to be very rare with only two articles published on such occurrences. Ritchie et al. reported three patients who developed LV systolic dysfunction 12–16 days after the completion of a 5 day induction of fludarabine infusions at a dose of 150 mg/m2 per day [6]. All three patients had recovery of their LV function 29–150 days after infusion. However, Newbery et al. reported a single patient who developed LV dysfunction after four infusions of fludarabine at a dose of 30 mg/m2 per day. This patient continued to have persistent LV dysfunction six months after completion of her regimen [4].
It is important to acknowledge that ATG has been associated with episodes of bradycardia. Bradycardia is recognized as a “rare” side effect in the manufacturer’s brochure, with an estimated occurrence of 1.5%. From our literature review of “antithymocyte globulin” and “bradycardia”, we were able to find four case reports (2 cases in children and 2 cases in adults) and a retrospective study in children reporting bradycardia after administration of ATG [16,17,18,19,20]. In all these cases, bradycardia was noted minutes to hours after the initiation of ATG. In this case presentation, however, the temporal relationship between the development of bradycardia and administration of ATG compared to the administration of fludarabine is important. The patient developed bradycardia 12 h after the second fludarabine infusion, which is 3 days after the completion of her ATG infusions. Bradycardia also resolved on the last day of fludarabine infusion. Thus, it seems more likely that bradycardia was secondary to fludarabine, rather than ATG. However, it is possible that ATG may have had a theoretical additive effect with fludarabine. With increased usage of ATG and fludarabine as conditioning agents for haploidentical cell transplantation in patients with sickle cell anemia and aplastic anemia, increased awareness of possible bradycardia with these two medications may be important [18, 19].
In general, fludarabine remains a relatively safe medication in respect to its effects on the cardiovascular system, with only a limited number of cases of adverse cardiac events published throughout the literature. Most of these reported cardiovascular adverse reactions are transient and usually do not require any additional therapy. Instead, patients typically have a good prognosis with a full recovery back to their baseline with close monitoring and supportive care [6, 8, 9].
Conclusion
Bradycardia is a relatively rare side-effect of fludarabine. We report the first case of ectopic atrial bradycardia associated with fludarabine. Close monitoring and supportive care are usually indicated. As fludarabine is ubiquitously used for a dramatically increasing number of patients receiving CAR T cell therapy for treatment of lymphoma and myeloma, clinicians should be aware of its potential for cardiac side effects.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- ATG:
-
Anti- thymocyte globulin
- BPM:
-
Beats per minute
- CAR:
-
Chimeric antigen receptor
- cGy:
-
Centigray
- EKG:
-
Electrocardiogram
- HSCT:
-
Hematopoietic stem cell transplantation
- LV:
-
Left ventricular
- MDS:
-
Myelodysplastic syndrome
- RV:
-
Right ventricle
- RVOT:
-
Right Ventricular Outflow Tract
- SA:
-
Sinoatrial
References
Wright SJ, Robertson LE, O’Brien S, Plunkett W, Keating MJ. The role of fludarabine in hematological malignancies. Blood Rev. 1994;8(3):125–34.
Lukenbill J, Kalaycio M. Fludarabine: a review of the clear benefits and potential harms. Leuk Res. 2013;37(9):986–94.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88.
Newbery G, Lima NA, Gurgel LA, Driscoll R, Lima CCV. Persistent heart failure following melphalan and fludarabine conditioning. J Cardiol Cases. 2019;20(3):88–91.
Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced Cardiotoxicity. Maedica (Bucur). 2013;8(1):59–67.
Ritchie DS, Seymour JF, Roberts AW, Szer J, Grigg AP. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant. 2001;28(1):101–3.
Peres E, Levine JE, Khaled YA, Ibrahim RB, Braun TM, Krijanovski OI, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(1):149–52.
Çelik S, Güven ZT, Altınsoy A, Tubay Ş, Keklik M, Ünal A. Fludarabine-induced bradycardia in allogeneic hematopoietic stem cell transplantation: A retrospective study. J Oncol Pharm Pract. 2023:10781552231189868.
Chung-Lo W, Hsieh CY, Chiu CF, Bai LY. Fludarabine-induced bradycardia in a patient with refractory leukemia. Hematol Oncol Stem Cell Ther. 2010;3(2):99–100.
Poręba M, Gać P, Usnarska-Zubkiewicz L, Pilecki W, Kuliczkowski K, Mazur G, et al. The analysis of the parameters of 24-hr ECG Holter monitoring in patients with blood neoplasms undergoing high-dose chemotherapy and stem cell transplantation. Ann Noninvasive Electrocardiol. 2018;23(4):e12534.
Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47.
Yusuf SW, Razeghi P, Yeh ET. The diagnosis and management of cardiovascular disease in cancer patients. Curr Probl Cardiol. 2008;33(4):163–96.
McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273–9.
Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MC, Donehower RC. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9(9):1704–12.
Chouchou F, Desseilles M. Heart rate variability: a tool to explore the sleeping brain? Front Neurosci. 2014;8:402.
Loushin MK, Hasinoff IK, Belani KG. A delayed cardiopulmonary reaction to an intravenous immunosuppressant thymoglobulin after pancreas transplant. Anesth Analg. 2001;93(5):1260–1 table of contents.
Kao SY, Xu W, Brandwein JM, Lipton JH, Messner HA, Minden MD, et al. Outcomes of older patients (> or = 60 years) with acquired aplastic anaemia treated with immunosuppressive therapy. Br J Haematol. 2008;143(5):738–43.
Godown J, Deal AM, Riley K, Bailliard F, Blatt J. Worsening bradycardia following antithymocyte globulin treatment of severe aplastic anemia. J Pediatr Pharmacol Ther. 2011;16(3):218–21.
Elazhary S, Alawyat HA. Bradycardia associated with antithymocyte globulin treatment of a pediatric patient with sickle cell disease: a case report and literature review. Hematol Transfus Cell Ther. 2022;44(2):284–7.
Kállay K, Zakariás D, Csordás K, Benyó G, Kassa C, Sinkó J, et al. Antithymocyte Globuline Therapy and Bradycardia in Children. Pathol Oncol Res. 2019;25(2):487–92.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SK, SN, and LZ collected patient data. SK, SN, LZ, DLC, and KJF interpreted patient data. SK, SN, LZ, and KJF analyzed EKG, echocardiogram, and Holter Monitor. SK, SN, and LZ performed literature reviews of fludarabine and bradycardia. SK and SN wrote the manuscript. DLC, KJF, and LZ critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patient provided consent and gave permission to have her case, as well as relevant related workup and diagnostic images, presented in the medical literature.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kong, S., Nagraj, S., Cooper, D.L. et al. A case report of fludarabine associated ectopic atrial bradycardia and literature review of fludarabine induced bradycardia. Cardio-Oncology 10, 50 (2024). https://doi.org/10.1186/s40959-024-00253-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00253-x